23+ Chapter 2 Basic Chemistry
Web a Carbon-12 12 C. Basic Chemistry In this Chapter.
Arrhenius Equation Calculator Online Solver With Free Steps
Web Biology 12th Edition answers to Chapter 2 - Basic Chemistry - Assess - Page 33 3 including work step by step written by community members like you.

. BANA 2082- Exam 4 study guide 2. Has an acid group b Glycine. Lesson 5 Plate Tectonics Geologys Unifying Theory Part 1.
Identify each of the following as a cation or an anion and determine the charge on each. Web Monmouth Regional High School District Homepage. Web 14 Study Guide for An Introduction to Chemistry Exercise 23 - Cations and Anions.
The correct answer for each question is indicated by a. Web Solved Chapter 2 Basic Chemistry 23 BIOCHEMISTRY. There are six electrons in a neutral 12 C atom.
Chapter 1 - Summary International Business. Web Basic Chemistry AP Biology Mader 11th Edition Chapter 2. The number of ___________ is always equal to the number of electrons in a.
B This atom contains six protons and six neutrons. Web 8-2 Project Three Systems Thinking. The net charge of such a neutral atom is zero and the.
The three isotopes of carbon 12 C 13 C and 14 C have different numbers of _______. Web A molecule of oxygen O 2 contains two oxygen atoms. Web Chapter 2 Outline Framework CONCEPTS OF MATTER AND ENERGY pp.
Which of the following elements is NOT one of the six that make up. Pre-Test Post-Test AnimationsVideosMP3 Learning Outcomes Chapter. Web Copyright 2010 Pearson Education Inc.
Basic Chemistry Learning Outcomes. After studying this chapter you should be able to accomplish the following outcomes. 22 States Solids Liquids Gases Changes Physical Level Chemical Level Energy pp.
Web a substance consisting of two or more different elements combined in a fixed ratio atom the smallest unit of matter that still retains the properties of an element. Web It has a pH of 70. Introduction In this chapter we start with a review basic chemistry from atomic structure to molecular bonds to the structure and properties of water followed by.
The subscript 2 in the formula must be used to distinguish the diatomic molecule from two single oxygen atoms. Figure 217 a Generalized a basic amino acidstructure of all is the simplest amino acids.
Browse Questions For Chemistry 101

Chapter 2 The Chemical Basis Of Life I Basic Chemistry A A

Rd Sharma Solutions For Class 7 Maths Chapter 23 Data Handling Ii Central Values Avail Free Pdf

Lecture Notes On Chemical Bonding Chemical Principles I Chem 110 Study Notes Chemistry Docsity
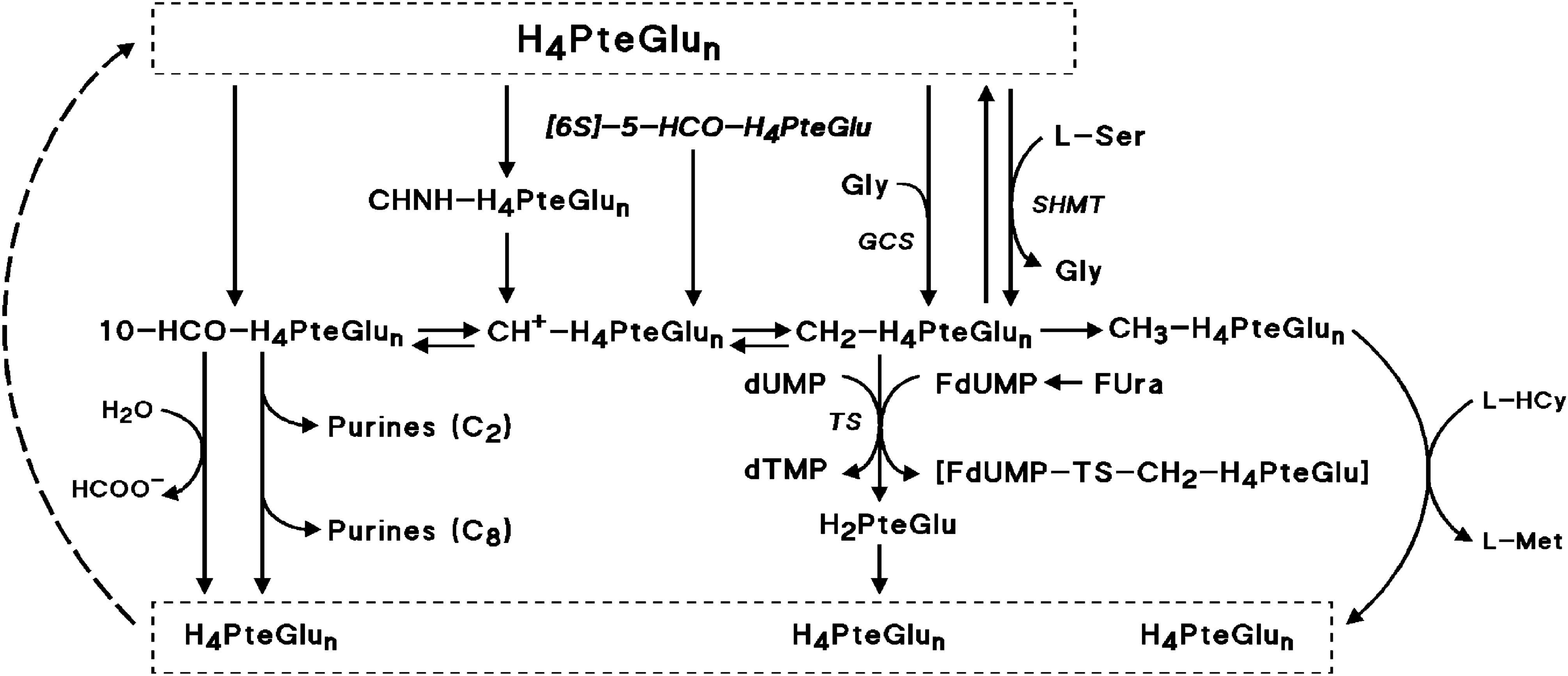
Pharmacologic Modulation Of 5 Fluorouracil By Folinic Acid And Pyridoxine For Treatment Of Patients With Advanced Breast Carcinoma Scientific Reports

Example 15 Factorise X3 23x2 142x 120 Class 9 Examples
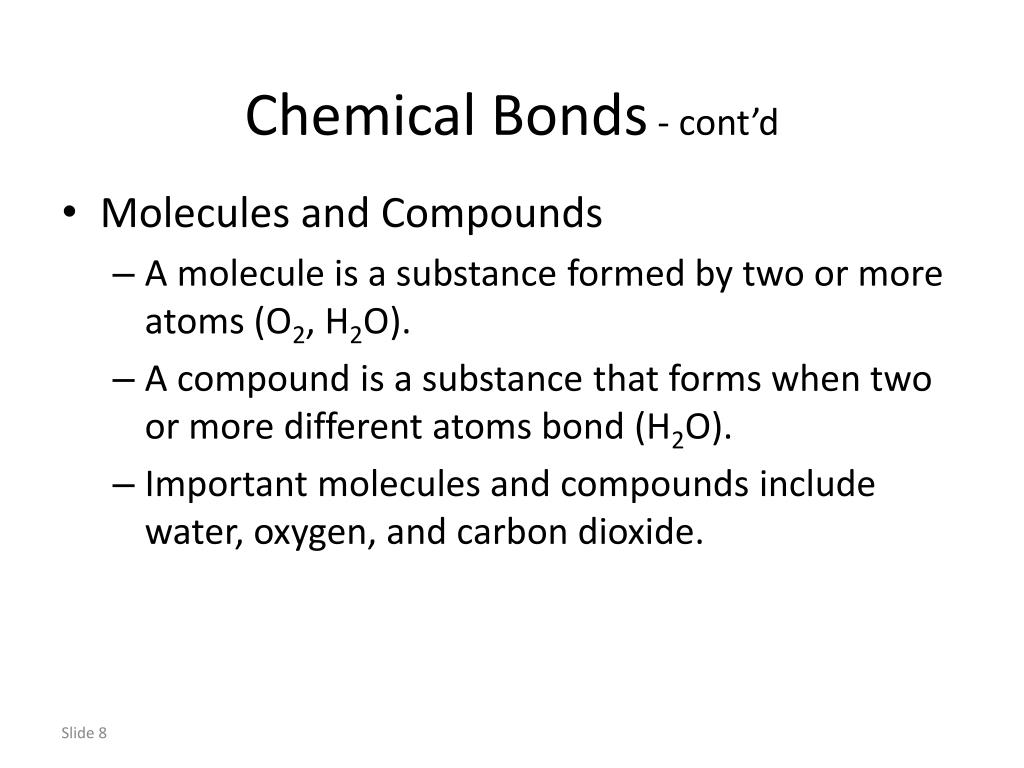
Ppt Chapter 2 Basic Chemistry Powerpoint Presentation Free Download Id 2413583
General Chemistry Notes Full Course Pdf Notes Chemistrynotes Com

Chemical Transformation Of Molecular Ices Containing N2o And C2d2 By Low Energy Electrons New Chemical Species Of Astronomical Interest The Journal Of Chemical Physics Vol 154 No 22
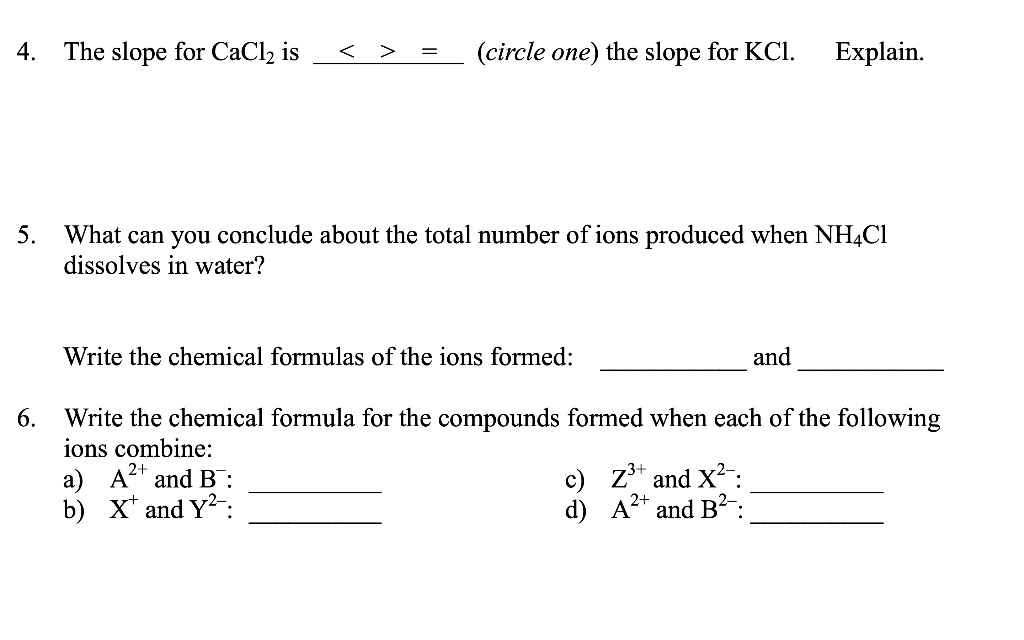
Solved 4 The Slope For Caclz Is Circle One The Slope For Kcl Explain 5 What Can You Conclude About The Total Number Of Ions Produced When Nhcl Dissolves In Water

Chemistry Quiz Teaching Resources Tpt

Chapter 2 The Chemistry Of Life Study Guide A Solution With A High

Chapter 2 Basic Chemistry

Chemical Bonding And Bonding Models Of Main Group Compounds Chemical Reviews

Chemistry 9th Edition Zumdahl Solutions Manual By Edzz Issuu

Ctla 4 Blockade Enhances Polyfunctional Ny Eso 1 Specific T Cell Responses In Metastatic Melanoma Patients With Clinical Benefit Pnas

Chapter 2 Basic Chemistry